Human monocyte, macrophage & dendritic cell assays
Monocytes are agranular leukocytes that originate from hematopoietic stem cells in the bone marrow. They circulate in the peripheral blood and are recruited to tissues following injury or infection. Once in the tissue, monocytes differentiate into inflammatory macrophages, which play a role in initiating the pro-inflammatory immune response and eliminating infectious pathogens.
Dendritic cells (DC) are known for their role as professional antigen presenting cells (APCs), along with macrophages and B cells. Upon exposure and uptake of pathogens, maturing cells travel to secondary lymphoid organs, and then become potent T cell activators. The upregulation of cytokines, chemokines, co-stimulatory molecules, and adhesion molecules by active DCs is a critical part of the adaptive immune response.
Our services include:
- Target expression profiling on subsets (resting vs. activated)
- Screening for modulation of M1/M2/MDSC (e.g., differentiation)
- Screening for modulation of tumor-conditional response (phenotype and cytokine/chemokine production)
- Effects on antigen presentation
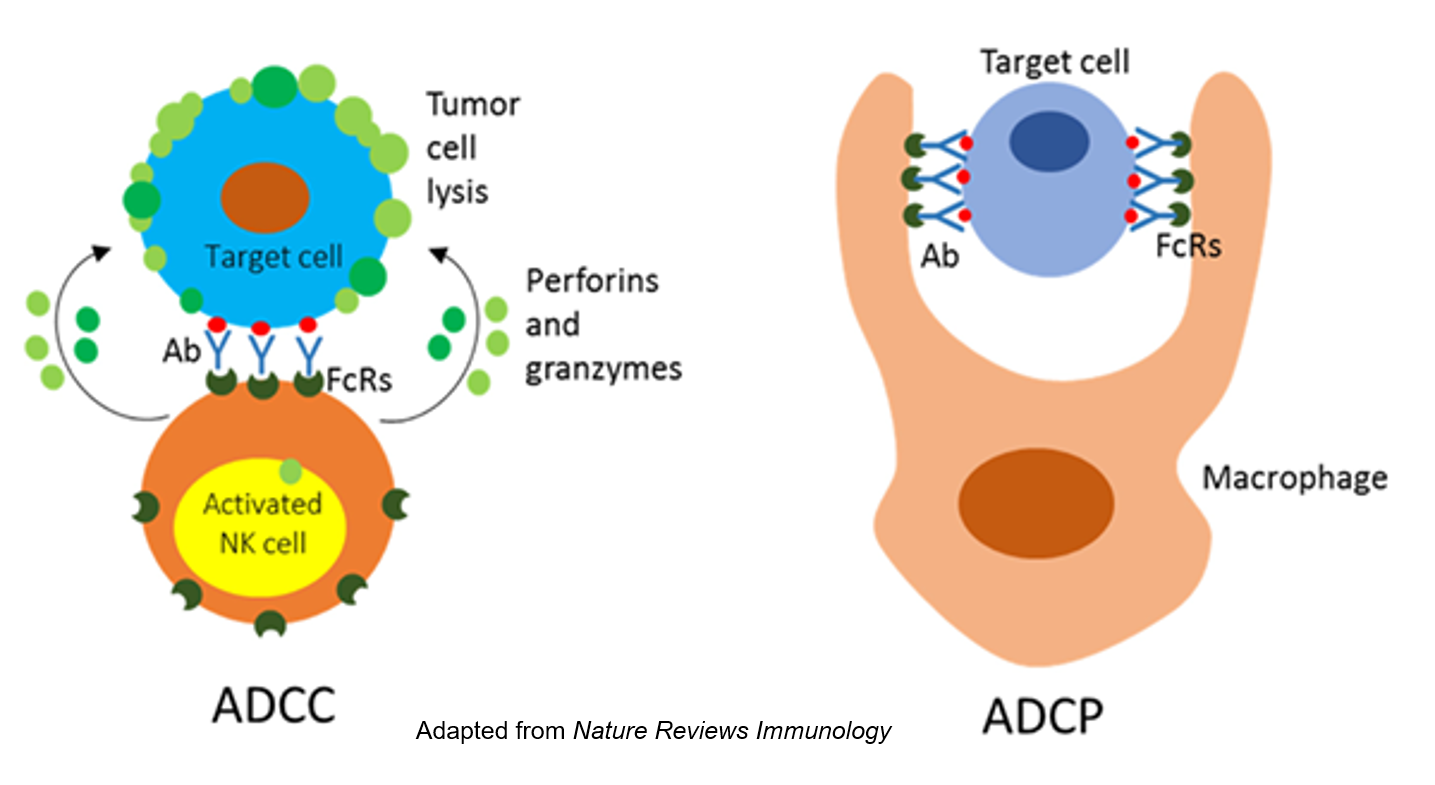
- Antibody-dependent cellular phagocytosis (ADCP)
- Monocyte activation test (MAT) for pyrogenic substances in pharmaceutical products
More about Monocyte Activation Test (MAT) assay
Pyrogenic substances in pharmaceutical products can induce life-threatening fever reactions after injection into the human body. Therefore, it is a regulatory requirement to test such products for pyrogens to ensure product quality and patient safety. The purpose of the test is to prove that the amount of pyrogens contained in the product will not exceed a certain threshold, known as the contaminant limit concentration (CLC), to guarantee the patient safety. The monocyte activation test (MAT) method has been among the compendial methods for pyrogen detection.
Principle of MAT assay
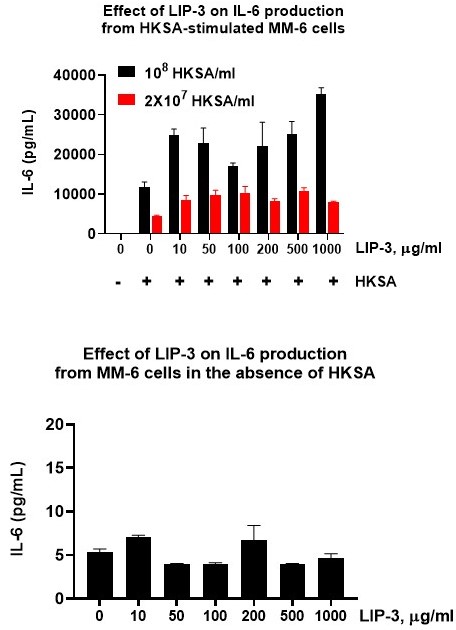
The monocyte activation test (MAT) is the human in vitro alternative to the rabbit pyrogen test (RPT), and allows the detection of the full range of pyrogens, including endotoxins and non-endotoxin pyrogens (NEPs). By putting the test product in contact with human monocytic cells, it mimics what happens in the human body: in presence of pyrogens, the monocytes are activated and produce several types of cytokines including IL-6. These cytokines are then detected using immunoassays.
PicoImmune MAT assay
Using cryopreserved pooled human PBMC or cryopreserved Mono-Mac-6 (MM6) human monocytic cells as a source of monocytes, we detect pyrogen impurities with our sensitive MAT assay on test samples. Our readout can be IL-6 or IL-1β ELISA and IL-6 or IL-1β FluoroSpot. The quantification includes an international standard of endotoxin and other non-endotoxin pyrogens (flagellin, HKSA, R848, PAM3CSK4) can be included in the assay.