About PhosphoFlow
Many proteins involved in cellular signaling cascades are phosphorylated. Fluorescently-labeled antibodies specific to a variety of phosphorylated proteins facilitate the detection of these important proteins at the single cell level with flow cytometry. Additionally, this assay allows for the simultaneous examination of multiple phosphoproteins in distinct cell subsets in samples with multiple cell types. This assay is a more powerful and sensitive approach than traditional assays such as Western Blots which only highlights global protein status in samples that can contain heterogenous populations.
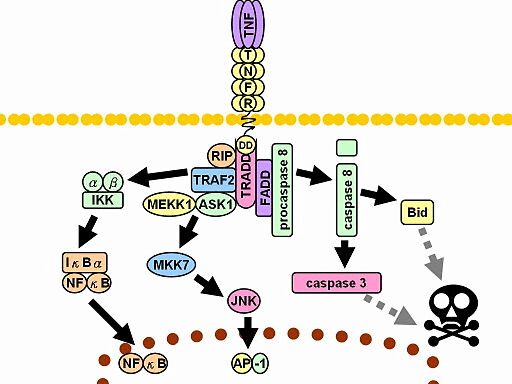
How Does the Assay Work?
Phosphoflow cytometric assays can specifically detect the state of protein phosphorylation at the cellular level. Our assays are optimized for multiple cell types including immune cells and whole bloods.
A general workflow is as follows:
- Treated and control cells or tissue/blood samples are processed and stained with viability dye and cell surface marker antibodies.
- The cells are fixed and permeabilized.
- The cells are then stained with the antibodies for intracellular phosphoproteins.
- The cells are acquired on a flow cytometer.
Sample Requirements:
- Minuscule samples (minimal volume)
- Compatibility with heterogeneous samples: works with a variety of sample types such as cultured cells, whole blood, PBMCs, isolated tissue cells (such as skin cells), etc.
Assay Benefits:
- Superior specificity: delivers greater specificity than other common technologies like Western blot, potentially revealing heterogeneity in samples.
- Flexibility: easily automated for use in routine compound screening
- Simultaneous detection: detect multiple targets (phosphoproteins) and other markers in the same cell type or in distinct cell subsets in a heterogeneous cell sample.
- Standardized platform: 96-well plate format using Cytoflex S flow cytometer.
- Use of specialized reagents: Assay conditions are optimized for accurate and reproducible data.
- Measure phosphorylation at a single cell
- Provides data for mechanistic insights.
Service Features:
- High throughput: 96-well format
- Simultaneous quantitation: detect phosphoproteins in multiple cell populations in the same sample and/or multiple phosphoproteins in the same cell type
- Highly reproducible
- State-of-art platforms: Cytoflex S flow cytometer
- Large inventory of phosphoprotein specific antibodies available
- Extensive data analysis
- Timely data delivery: 1-4 weeks or sooner, dependent upon receiving test samples
- 20+ years of experience: Expert data analysis and interpretation, high quality scientific and technical support
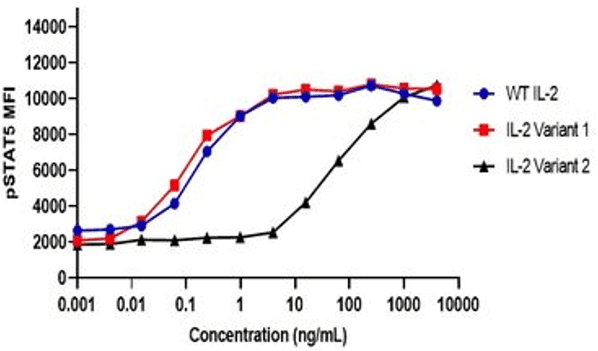
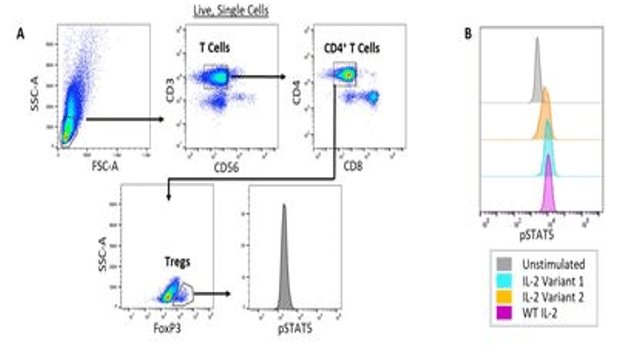
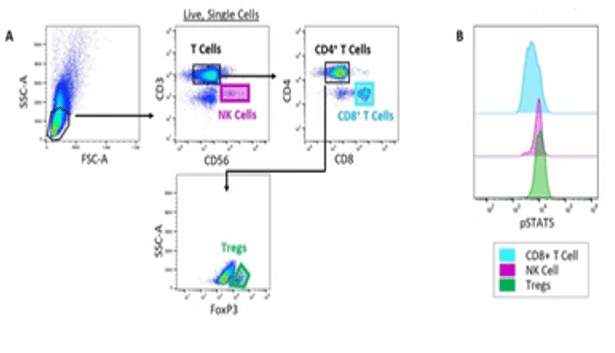
Example Data